In general, physical structures can be divided into two categories — giant structures and simple structures.
Simple molecular structures consist of atoms or molecules bound by intermolecular forces arising from hydrogen bonding and dipole-dipole interactions. Giant structures are formed are atomic networks strong bonded to each other. The most common types of giant structures are metals, ionic solids, polymers, ceramics and glasses. Their properties rely on three types of strong bonding – metallic, ionic and covalent. All bonds are electrostatic in nature, and their strength depends on electrostatic attractions between the nuclei and surrounding electrons.
Metallic Bonding
Metallic bonding emerges from the electrostatic attraction between the electron cloud of delocalised electrons and metal cations. Diffraction patterns of metals provide evidence for the general tendency of cations towards close packing, such as body centered and face centered cubic arrangements.
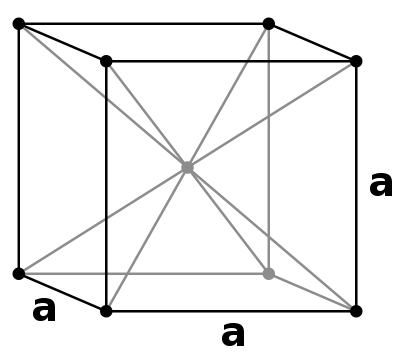 | 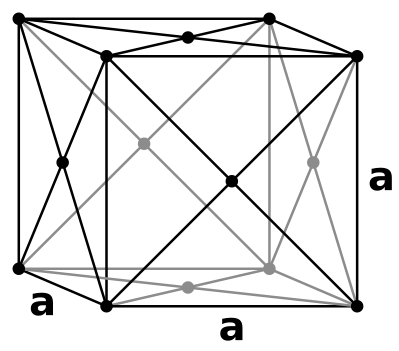 |
| body centered cubic (bcc) | face centered cubic (fcc) |
Metals are electron deficient due to the partially filled electron shells. If an external electric field is applied, the delocalised sea of electrons moves freely through the structure, which explains high electrical conductivity of metals. Metals also exhibit high thermal conductivity due to the vibrations, known as phonons, of the high energy electrons participating in the heat transfer. If the temperature increases, more heat can be transferred by the metal, while the electrical conductivity decreases due to the agitation of the electrons resisting the flow induced by the electric field. Other characteristic properties,ductility and malleability, arise from the ability of cation layers to slip past each another without breaking.
Thus, metal atoms in solid state are arranged in giant structures with strong bonds between the positive metal ions and a sea of delocalised electrons, which account for high melting and boiling points, high density, and great electrical and thermal conductivity.
Ionic Bonding
When electrons are transferred from one atom or molecule to another, positive and negative ions form. Ions of positive charges attract each other, forming giant ionic structures.
Why do ions form one charge and not the other?
Energy is required to remove electrons from an atom or molecule. For a mole of atoms in the gaseous state, the ionisation energy is defined as the minimum amount of energy to remove one mole of electrons from the closest electron shell. The more electrons are removed from the species, the greater ionisation energy is required.
Atoms and compounds interact with each other and form bonds to attain the most stable energy state. When the bonds form, energy is released. As a rule of thumb, the greater energy released from the formation of the bonds, the more stable the compound is. In these words, energy and entropy considerations dtermine the most stable ionic configurations.
Ionic compounds are giant structures with cations and anions attracting each other. For instance, the crystal of NaCl can be described as an aggregate of sodium cations and chloride anions with each ion bonded to its six neighbours of opposite charge.
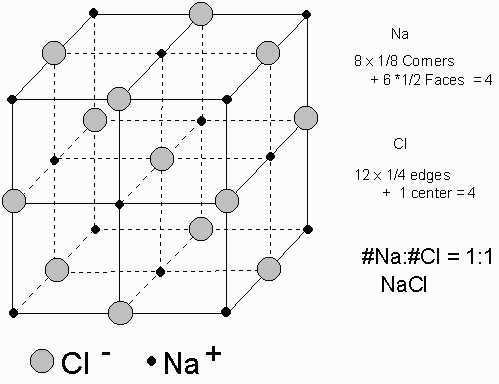 |
Ionic substances dissolve only in polar solvents (e.g. water, dichloromethane, ethyl acetate). In the first decades of the XX century it was noticed that some liquids have a small dielectric constant nearly independent of the temperature, while in the others the dielectric constant decreases rapidly as temperature rises. The concept polar and non-polar was developed to explain the phenomenton. Liquids of the first kind, called non-polar solvents, consist of molecules with no electric dipole moment. Liquids of the second kind, called polar solvents, consist of molecules which form a permanent dipole-dipole moment.
Ionic compounds do not dissolve in non-polar solvents, since there are no electrostatic attraction between the neutral molecules of the solvent and the solute. Water is a polar molecule; electronegative oxygen atom bears partial negative charge, while two hydrogen atoms form the positive side of the dipole. Thus, upon dissociation, cations of the ionic solute bond to the negative pole of the water molecule, while anions attract partially positive hydrogens. The giant ionic structure dissociates, and mobile ions become hydrated. A liquid which contains these mobile ions is called an electrolyte If a potential difference is applied to the solution, cations drift to the negative cathode where they are reduced, while anions move towards the positive anode and become oxidised. An electrolyte can also be produced by melting an ionic compound.
Although molten or dissolved ionic compounds are good electricity conductors, they are usually insulators in the solid state. Since there are no free electrons moving in the solid ionic compound, no carriers of charge are present. However, ionic conductivity can be substantial, if ionic solids contain defects, allowing migration of ions, or mobile interstitials. Moreover, some compounds, such as silver iodide, have high electric conductivity in the solid state due to the partial covalent character of the structure. The crystal of AgI is cubic, with four iodide ions in the unit cell arranged in the close-packed positions (0, 0, 0), (0, 1/2, 1/2), (1/2, 0, 1/2), (1/2, 1/2, 0). The x-ray diffraction patterns show that the silver ions assume octahedral and tetrahedral positions. Silver ions can alter their position upon heating to 146 °C. When heated, the iodide ions form a rigid cubic framework with molten silver ions whizzing inside.
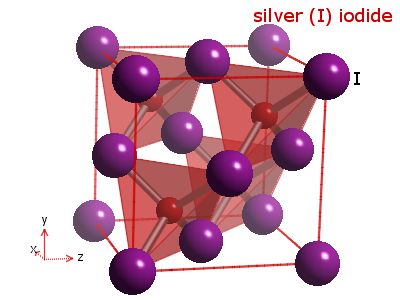 |
In conclusion, ionic compounds are giant crystals which are soluble in polar solvents and conduct electricity if dissolved or molten.
Covalent Bonding
Covalent bonding involves electron sharing between atoms of a covalent compound. The atoms are held together by the electrostatic attraction between the positive nuclei and the negative electron cloud. Intermolecular interactions are relatively weak, and thus simple covalent structures are most often liquids or gases at room temperature with relatively low melting and boiling temperatures.Covalent bonds are directed and thus act in a particular direction determined by their spatial orientation. Giant covalent structures are predominantly carbon, silicon or boron based. Carbon atom, with four valence electrons in the outer shell, can form four covalent bonds. These bonds combine into various distinct structures, known as allotropes. One of the well known allotropes of carbon is a diamond, in which each atom is bonded strongly to four neighbouring atoms arranged in tetrahedral positions. Since all electrons are involved in bonding and there is no electron deficiency, diamond does not conduct electricity. Strong carbon-carbon covalent bonds explain characteristic hardness and insolubility of diamond in common solvents.
 |  |
Another well known allotrope of carbon is graphite. Graphite consists of layers of carbon atoms. Each atom has three neighbours. The p-orbitals of carbon atoms with valent electrons overlap and form a sea of delocalised electrons.Since the electron sea is free to move through the single layer of graphite, but not from one layer to another, graphite anisometrically conducts electricity. Sheets of carbon are held by weak dispersion forces and can easily slide past each other, accounting for the greasy texture of the material. Graphite has a high melting point and is used as a thermal insulator which prevents melting of industrial furnaces. Graphite is also insoluble in water and organic solvents due to the covalent character of the structure.
 |
If graphite layers are peeled off, or, in other words, if graphite is exfoliated multiple times, transparent one atom thick sheet of carbon atoms, called graphene, is produced. Graphene was first produced in the lab in 2003. Due to the 2 dimensional structure of graphene and valency of the lateral carbon atoms, graphene is the most reactive carbon allotrope. Graphene retains the giant structure and properties of graphite, such as high thermal and electrical conductivity. If a sheet of graphene is rolled up in a tube at specific angles, carbon nanotubes are formed. The combination of the rolling angle and the resulting radius determines the nanotube properties
 |  |
| a sheet of graphene on paper |
In 1985, a third allotrope of carbon was discovered, known as a buckminsterfullerene or buckyball. The name 'buckminster fullerene' comes from the architect and inventor of the geodhesic dome, Richard Buckminster Fuller. Buckminsterfullerene, C60, has a form of a truncated icosahedron, resembling a soccer ball, made of 20 hexagons and 12 pentagons. Buckyballs, in contrast to graphite and diamonds, have a simple molecular structure and a well-defined molecular formula. The open surface of the structure can selectively react with other species while preserving its spherical geometry. On the other hand, atoms and small molecules can be entrapped in the interior with no chemical reaction taking place.
| buckminsterfullerene |
Buckminsterfulllerenes have a lower melting point than diamonds and graphite; C60 sublimes at 800K, while diamond and graphite sublime at 4000K. It is insoluble in water, since carbon atoms form only weak Van der Waals attractions with water molecules. However, it is soluble in organic solvents, such as benzene. Pure buckminsterfullerenes are electrical insulators, since the delocalised electrons on the surface cannot move from one buckyball to another. Nevertheless, by doping buckyballs with other species they can be turned into semiconductors or conductors.
Both carbon nanotubes and buckyballs are members of the wide class of carbon materials, fullerenes.
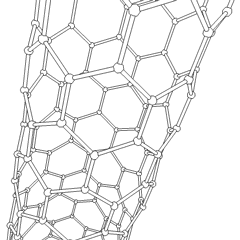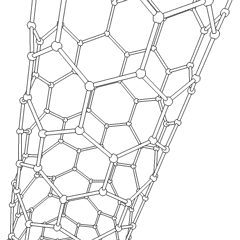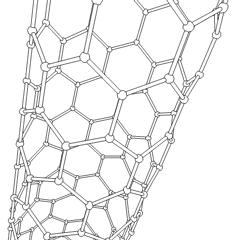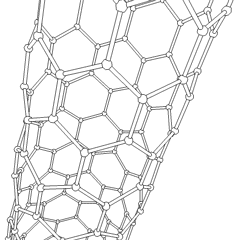 | 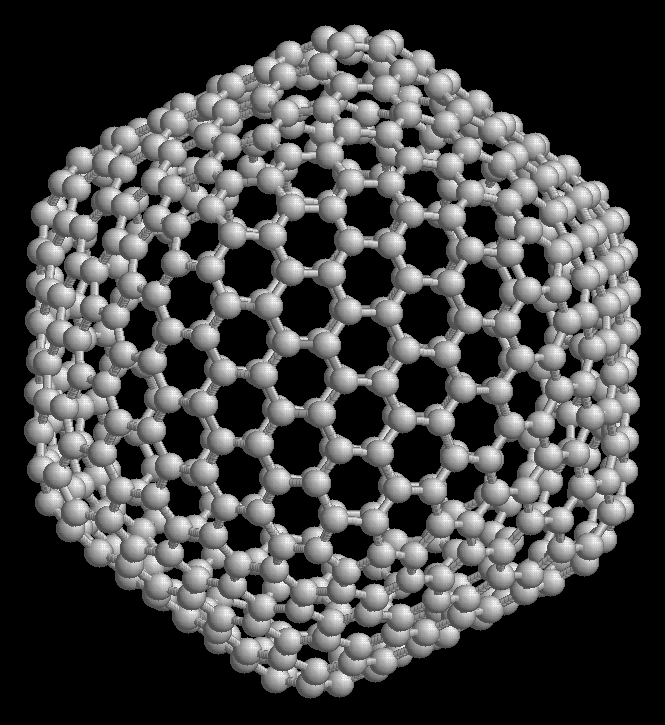 |
| rotating carbon nanotube model | the icosahedral fullerene, C540 |
Another compound with a giant covalent structure is silicon dioxide, also known as silicon(IV) oxide or silica.
 | 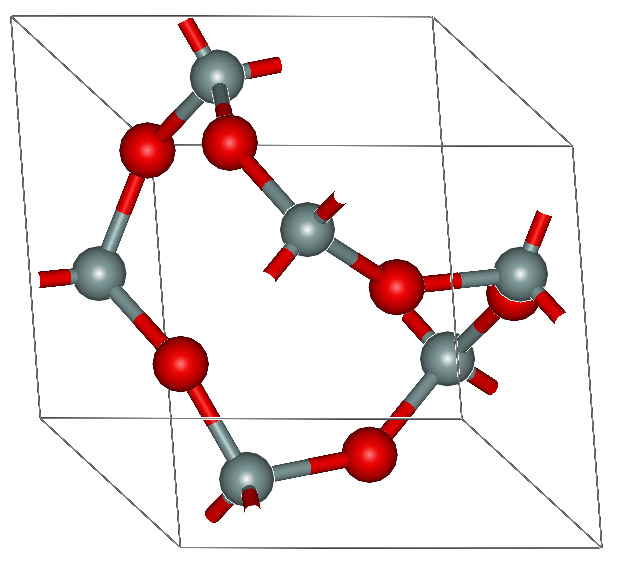 |
| silica crystal, quartz | structure of α-quartz |
There are three major types of silicon dioxide crystals: quartz, tridymite and cristobalite. The angles between the tetrahedral arrangements of silicon and oxygen change upon heating, and the crystals transform into one another as temperature rises. The high melting point and characteristic hardness of silicon dioxide is due to the strong covalent bonds between the atoms of oxygen and silicon. Silica does not conduct electricity in its crystalline form, since there are no delocalised electrons or electron deficiency, and is insoluble in water and organic solvents.
In conclusion, under standard conditions giant structures are strong,tough and hard frameworks of atoms with high melting and boiling points. Simple molecules are held together by relatively weak dipole-dipole moments and exhist in either gaseous or liquid state.
REFERENCES
1. Edexcel Chemistry for AS by Graham Hill, Andrew Hunt (ISBN: 9780340949085)
2 General Chemistry (Dover Books on Chemistry) by Linus Pauling (ISBN: 9780486656229)
3. http://ww2.chemistry.gatech.edu/class/6182/wilkinson/ionic.pdf , retrieved on 9/11/2014
4. http://en.wikipedia.org/wiki/Fast_ion_conductor#Superionic_conductors , retrieved on 9/11/2014
5. http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html#c2 , retrieved on 9/11/2014
6. Geim, A. (2009). "Graphene: Status and Prospects". Science 324 (5934): 1530–4.
7. http://ibchem.com/IB/ibnotes/full/bon_htm/14.4.htm , retrieved on 9/11/2014
8. http://www.chemguide.co.uk/atoms/structures/giantcov.html , retrieved on 9/11/2014
9. http://www.chemguide.co.uk/CIE/section113/learninge.html , retrieved on 9/11/2014
10. http://apps.kemi.se/flodessok/floden/kemamne_eng/kiseldioxid_eng.htm , retrieved on 9/11/2014
11. http://chemwiki.ucdavis.edu/Wikitexts/UC_Davis/UCD_Chem_124A%3A_Kauzlarich/ChemWiki_Module_Topics/The_Unit_Cell , retrieved on 9/11/2014
12. http://www.earthsci.org/education/teacher/basicgeol/miner/nacl.gif , retrieved on 9/11/2014
13. http://www.webelements.com/_media/compounds/Ag/Ag1I1-7783962.jpg , retrieved on 9/11/2014
14. https://betterthandiamond.com/images/diamonds/asha/round/v5_round_diamond_asha_med.jpg , retrieved on 9/11/2014
15. https://www.uwgb.edu/dutchs/Graphics-Geol/ROCKMIN/ATOM-STRUCT/Diamond3.gif , retrieved on 9/11/2014
16. http://upload.wikimedia.org/wikipedia/commons/f/f3/GraphiteUSGOV.jpg , retrieved on 9/11/2014
17. http://upload.wikimedia.org/wikipedia/en/1/19/Graphene_visible.jpg , retrieved on 9/11/2014
18. http://cnx.org/resources/bac5ec5fc6a10f049e0f85c5470c7d24/graphene.jpg , retrieved on 9/11/2014
19. http://upload.wikimedia.org/wikipedia/commons/b/bf/Buckminsterfullerene.svg , retrieved on 9/11/2014
20. http://upload.wikimedia.org/wikipedia/commons/1/14/Quartz,_Tibet.jpg , retrieved on 9/11/2014
21. http://upload.wikimedia.org/wikipedia/commons/5/53/A-quartz.png , retrieved on 9/11/2014
21. http://upload.wikimedia.org/wikipedia/commons/7/76/Kohlenstoffnanoroehre_Animation.gif , retrieved on 9/11/2014
22. http://upload.wikimedia.org/wikipedia/commons/c/c5/Fullerene_c540.png
Alexander I.
9/11/2014
